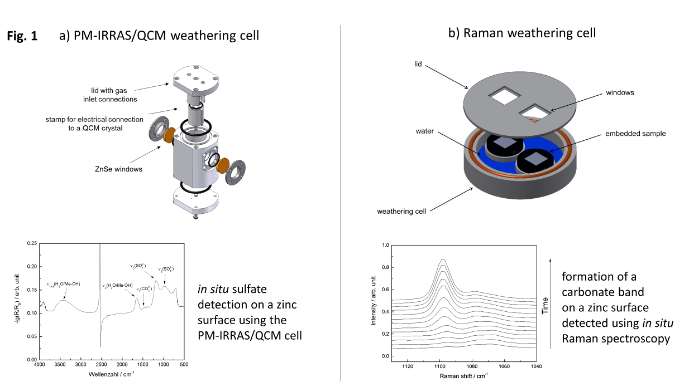
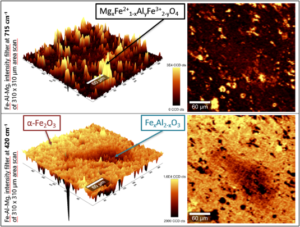
Area 2 – Corrosion
Corrosion is a ubiquitous phenomenon, which is of great importance for the economy, since every year values of about 5% of GDP are destroyed by corrosion. Especially with safety critical structures like bridges a precise understanding of how to prevent corrosion is important. Corrosion research therefore has a high relevance for the economy and society.
Corrosion of metallic materials preferably occurs under conditions where a conductive substance is brought into contact with a corrosive medium. Under such conditions, electrochemical reactions can take place on the surface which lead to the steel being attacked. In the worst case the function of the component is no longer given and the total failure of the component can occur.
Since its foundation in 2000, CEST has intensively dealt with these questions, in particular with the elucidation of corrosion mechanisms as well as with the development of new coatings of both organic and inorganic nature (e.g. ZnCr alloy layers on galvanized steel, plasma electrolytically produced layers on aluminum or titanium). The development of efficient and environmentally friendly processes for corrosion protection helps to save costs and is of utmost importance for safety, health and environmental protection. In particular, the replacement of heavy metal or environmentally harmful and toxic surface treatments of metals, for example the search for alternatives to chromium (VI) conversion coatings, is a major challenge.
For the investigation of corrosion processes, CEST uses proven techniques such as electrochemical impedance spectroscopy (EIS) or salt spray tests as well as novel methods (such as corrosion detection by artificial intelligence).
We not only carry out research, but are also partners for companies in the field of corrosion. We carry out corrosion tests in accordance with the necessary standards, investigate cases of damage and are happy to assist our customers when it comes to necessary measures for the prevention and detection of corrosion.
In recent years, we have built up special expertise on a number of topics: